U.S. approves HIV prevention drug with six-month protection
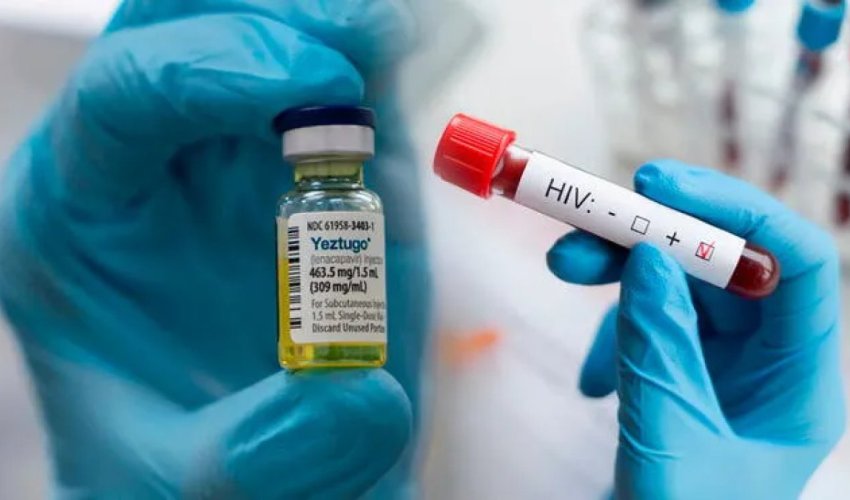
The U.S. Department of Health and Human Services has approved a new HIV prevention drug, Yeztugo, developed by Gilead Sciences, which provides protection for up to six months, Kommersant reported.
The drug, a capsid inhibitor, blocks the virus from entering human cells. It has passed clinical trials and was named Breakthrough of the Year by Science magazine in 2024.
Gilead has begun registering Yeztugo in North America, South Africa, Brazil, Switzerland, and other countries. Under an agreement with the Global Fund to Fight AIDS, the drug will be supplied royalty-free to 120 low- and middle-income countries. Up to 2 million people are expected to receive it within three years.
In the United States, Yeztugo will be provided free of charge to eligible individuals through a Gilead access program.
Experts say the twice-yearly injection could overcome key barriers of previous HIV prevention methods, such as the need for daily pills and associated social stigma.
Latest news 
More news 



































 Photo
Photo 



 Video
Video